Empowering Seamless Access: Personalised Pharmacogenomic Information On The Go
In a study done by Agency for Science, Technology and Research (A*STAR) and Singapore General Hospital (SGH), about 30% of adverse drug reactions (ADRs) admitted to SGH were caused by at least one drug with a clinical annotation in the Pharmacogenomics KnowledgeBase (PharmGKB). Furthermore, research findings from the SG10K_Health study further revealed that 26.8% of Singaporeans carry a genetic variant that raises the risk of life-threatening side effects to at least one medication.
We spoke to NalaGenetics’ Market Access Lead, Dr Kamonlawan Chomchopbun, on how the company is changing this narrative and the team’s aspirations for the technological solutions which they are developing.

How did NalaGenetics come to work with the National Precision Medicine (NPM) programme?
NalaGenetics was founded in 2016 with the development and commercialisation of our first flagship genetic test kit for leprosy. But we are always on the lookout for opportunities to leverage our knowledge in pharmacogenomics, genetic testing and equivalent technologies to positively impact more lives in Southeast Asia. We wanted to champion a cause that resonates with us. When we learned about the launch of Phase II of the NPM programme in 2021, we saw an opportunity to make a difference to the Singapore healthcare system with our know-how—so of course we wanted to be a part of it!
That’s how we began discussions about collaborating with Precision Health Research, Singapore (PRECISE) who is driving Phase II of NPM. We noted a gap between patients and the healthcare providers—patients had little knowledge and awareness about pharmacogenomics, which in turn limited its adoption into clinical settings in the Singapore healthcare system. NalaGenetics proposed to bridge this gap with our mobile app, which enables patients to access their pharmacogenomic information and share with their healthcare providers.
Interesting—what is NalaGenetics trying to solve with the mobile app?
Currently, pharmacogenomics is not widely rolled out in the Singapore healthcare system even though there is evidence of better clinical outcomes and lower healthcare costs. This is because patients often do not remember or fully grasp the implications of their pharmacogenomics data. At the same time, the diversity and complexity of the healthcare sector meant that not all healthcare providers had access to the system that stores the patients’ information.
We were prompted to answer the big questions—how can we free patients from the burden of having to remember what medication they can or cannot take? How can we empower doctors to be sure that the medicines they are prescribing will not cause an ADR?
We found the answer in—a mobile app. Handy, easily accessible and with possibilities of sharing secured data, patients can not only securely access their pharmacogenomics information during medical consultations, but also share it with their doctors.
Can you share more about the mobile app?
We rolled out our first prototype in August 2022 after the completion of user testing. While we have made a few adjustments to the app such as allowing users to login via SingPass and adding more medications to the panel, the core function and features of the mobile application have mostly stayed the same.
Essentially, registered users who have completed their pharmacogenomics testing just need to download the Nala Personal Health Manager app from either Google Play or Apple App Store . Thereafter, they can securely log in to access their personalised pharmacogenomics data once the lab test results are available. But the value of the app doesn’t end there.
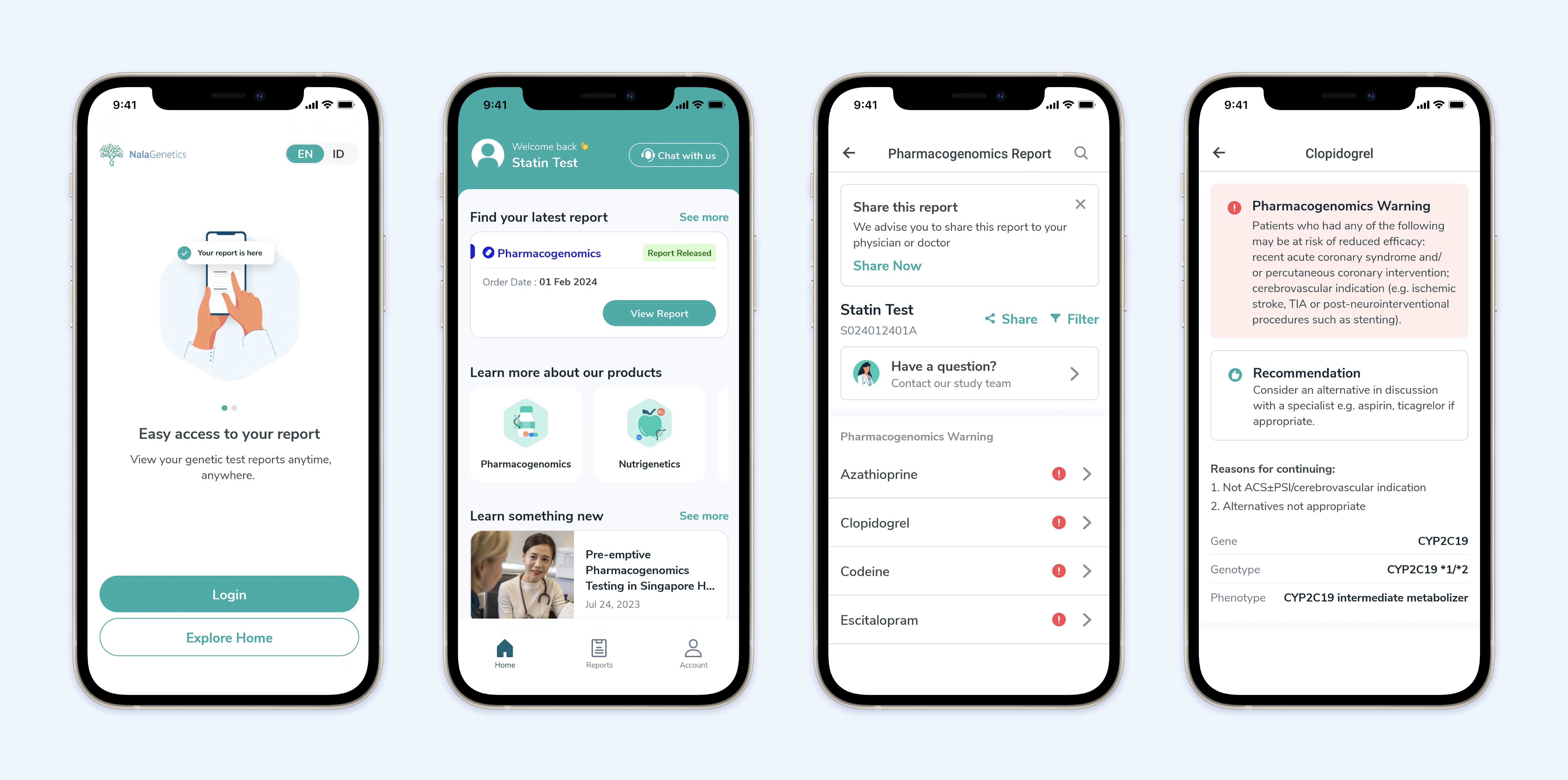
Instead, its significance lies in enabling users to share this information with doctors. There is no need for users to remember details—the system remembers it for you and reminds you with an instant pop-up upon logging in. Then to share it with the doctors, the users can either just show their doctors the information on their mobile, generate a QR code for the doctors to scan and log in, or email the link to the doctor for retrieval.
How has the collaboration with PRECISE and the NPM been for NalaGenetics thus far?
PRECISE has been very supportive. Through their advocacy and network, we connected and collaborated with various passionate and like-minded scientists, clinicians, hospitals and other industry partners—important for ensuring that the solutions NalaGenetics are building remain feasible, practical, and seamlessly integrated into clinical settings.
NalaGenetics has experience in genetic testing and pharmacogenomics—so we have insights into the workings of the healthcare industry and a good understanding of doctors’ pain points when it comes to adopting pharmacogenomics. Different institutions and electronic medical records system setups meant that information is stored independently. Doctors do not have the same level of access to information. On top of that, busy schedules amplify the importance of presenting information clearly and concisely. Particularly, for doctors with access to patients’ pharmacogenomics information, they should see the same thing as what is shared on the Nala Personal Health Manager app to prevent confusion.
In order to shape our technology solution to address these challenges, we interviewed various stakeholders from public and private hospitals as well as providers in primary healthcare. The process was rigorous, but also very heartening—because everyone contributed their time and knowledge generously towards the solution. We have now advanced the development of the platform to the user trial phase—where we are recruiting patients for pharmacogenomics testing and onboarding to the Nala Personal Health Manager app.
What can we look forward to in the future for the application and NalaGenetics?
Presently, the application is at a stabilised state. Key features are in place, data security standards are met, approximately 30 medications with significant clinical impact, widespread use, and potential for serious adverse reactions associated with genetic variability are included in the panel—a four-fold increase from the first prototype. We will look to grow the number of medications in the panel further.
The application offers a good opportunity for pharmacogenomics testing to go from reactive to pre-emptive. After all, pharmacogenomic test results are valid for life—and now they can also be securely stored and easily accessible for life. As a company that prioritises patient safety, this is definitely an initiative we support.
Another area that NalaGenetics hopes to lead in is advancing pharmacogenomics knowledge for the Southeast Asian population. What we know about genetics today are mostly derived from research conducted in US and Europe. While they are useful in providing a basis for our understanding, we cannot be sure that genes in the local population interact with these medicines the same way. Hence, with the data we collect on our application, we hope to empower clinical actionability.
Beyond these, we are deeply inspired by our interactions with many of these collaborators. We are excited to explore how we can create more partnership opportunities with them.
1Chan SL, Ang X, Sani LL, Ng HY, Winther MD, Liu JJ, Brunham LR, Chan A. Prevalence and characteristics of adverse drug reactions at admission to hospital: a prospective observational study. Br J Clin Pharmacol. 2016 Dec;82(6):1636-1646. doi: 10.1111/bcp.13081. Epub 2016 Sep 19. PMID: 27640819; PMCID: PMC5099543
2 https://www.cris.sg/news-and-events/media-releases/230220-precise-sg10k-study/

